How do crystals form?
- Posted on
- By Bart Verboven
- Posted in crystal, Crystallography, crystals, Mineral, minerals, natural crystals, quartz

Crystals come in the most beautiful shapes and colours, but how do they form? And what is the largest crystal ever found? We take a look into the wonderful world of crystallography.
Table of Contents
- The definition of crystals
- Crystallography
- Repetition of patterns
- Perfect crystals
- Beautiful colours
- Growing crystals
- Symetry
- The peculiar properties of quartz crystals
- Impurities
- The biggest crystals in the world
The definition of crystals
 Before we go deeper into the wonderful world of crystals, we first make a definition of what a crystal is: A crystal is a solid material whose components, such as atoms, molecules or ions, are arranged in a highly ordered structure, forming a crystal lattice that extends in all directions. Many crystals are characterised by a geometric shape, consisting of flat surfaces with specific, characteristic orientations.
Before we go deeper into the wonderful world of crystals, we first make a definition of what a crystal is: A crystal is a solid material whose components, such as atoms, molecules or ions, are arranged in a highly ordered structure, forming a crystal lattice that extends in all directions. Many crystals are characterised by a geometric shape, consisting of flat surfaces with specific, characteristic orientations.
Crystallography
 The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation through mechanisms of crystal growth is called crystallization or coagulation. The word crystal is derived from the Ancient Greek word κρύσταλλος (krustallos), which means both "ice" and "crystal".
The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation through mechanisms of crystal growth is called crystallization or coagulation. The word crystal is derived from the Ancient Greek word κρύσταλλος (krustallos), which means both "ice" and "crystal".
Repetition of patterns
 Most minerals are found in a crystal form. Each crystal has an ordered, internal atomic pattern, with a distinctive way of locking new atoms into that pattern and repeating it over and over again. The shape of crystals can vary considerably. Think for example of a cube (such as pyrite) or a hexagon (such as a snowflake). These shapes reflect the internal arrangement of the atoms. As crystals grow, differences in temperature and chemical composition cause fascinating variations. Crystals are therefore popular in jewelry.
Most minerals are found in a crystal form. Each crystal has an ordered, internal atomic pattern, with a distinctive way of locking new atoms into that pattern and repeating it over and over again. The shape of crystals can vary considerably. Think for example of a cube (such as pyrite) or a hexagon (such as a snowflake). These shapes reflect the internal arrangement of the atoms. As crystals grow, differences in temperature and chemical composition cause fascinating variations. Crystals are therefore popular in jewelry.
Perfect crystals
 Perfectly shaped crystals are rarely found. In order for the geometric shape and flat surfaces to grow perfect, the crystals also need ideal growing conditions. When, for example, many different crystals grow close together, they form a kind of fused mass with hardly any crystal formation. This is the case with rocks such as granite, which consists of many small mineral crystals. In order to grow into a perfect crystal, it needs a lot of space without competition.
Perfectly shaped crystals are rarely found. In order for the geometric shape and flat surfaces to grow perfect, the crystals also need ideal growing conditions. When, for example, many different crystals grow close together, they form a kind of fused mass with hardly any crystal formation. This is the case with rocks such as granite, which consists of many small mineral crystals. In order to grow into a perfect crystal, it needs a lot of space without competition.
Beautiful colours
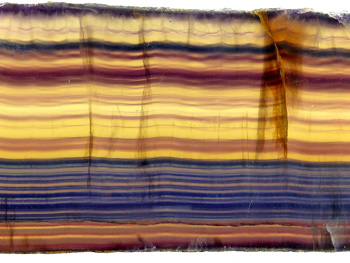 The internal arrangement of the atoms determines the chemical and physical properties of minerals including their colour. However, light and radiation can influence this, which can lead to a diversity of colours. Many minerals are colourless in their pure state, where radiation or light gives colour to the crystal. Quartz in its pure state is colourless, but is found in a range of colours. From pink, yellow and brown to deep purple amethyst and combinations thereof. In its colourless state quartz resembles ice. The ancient Greeks believed that clear quartz was frozen ice that could not melt. The most colorful mineral is fluorite. These crystals are also colourless in pure form, but react strongly to light and radiation during its growth. In some crystals coloured bands can be found that indicate altered light conditions during the formation.
The internal arrangement of the atoms determines the chemical and physical properties of minerals including their colour. However, light and radiation can influence this, which can lead to a diversity of colours. Many minerals are colourless in their pure state, where radiation or light gives colour to the crystal. Quartz in its pure state is colourless, but is found in a range of colours. From pink, yellow and brown to deep purple amethyst and combinations thereof. In its colourless state quartz resembles ice. The ancient Greeks believed that clear quartz was frozen ice that could not melt. The most colorful mineral is fluorite. These crystals are also colourless in pure form, but react strongly to light and radiation during its growth. In some crystals coloured bands can be found that indicate altered light conditions during the formation.
Growing crystals
 Scientists usually describe crystals as "growing" even though they are not alive. In underground cavities, crystals grow through atoms that connect in regular three-dimensional patterns. Each crystal starts small and grows as more atoms are added. Many grow in water that is rich of dissolved minerals. However, this is not a condition, crystals can also grow from molten rock or even fumes. Under the influence of temperature and pressure, atoms combine to form an amazing range of crystal shapes. It is this variety and perfection of shape and symmetry that has attracted scientists to the study of minerals for centuries.
Scientists usually describe crystals as "growing" even though they are not alive. In underground cavities, crystals grow through atoms that connect in regular three-dimensional patterns. Each crystal starts small and grows as more atoms are added. Many grow in water that is rich of dissolved minerals. However, this is not a condition, crystals can also grow from molten rock or even fumes. Under the influence of temperature and pressure, atoms combine to form an amazing range of crystal shapes. It is this variety and perfection of shape and symmetry that has attracted scientists to the study of minerals for centuries.
Symetry
 Symmetry is a regular, repeated pattern of parts and can be found everywhere in nature. Think of the wings of a butterfly, the seeds and leaves in a sunflower or the pattern of a snowflake. Minerals are no exception. In crystals, the shape is determined by the repeated atomic patterns and reflect the 'face' of the crystal. You often see the characteristic symmetry of a crystal with the naked eye, but if the crystal is very small, then a magnifier or magnifying glass is a convenient instrument. Recognizing symmetrical patterns in crystals seems complicated, but experience helps. The more crystals you have seen, the better you can recognize the symmetry. However, sometimes the crystal is far from perfectly shaped and difficult to classify even for experts. In science, crystals are therefore defined by atomic arrangement and not by appearance.
Symmetry is a regular, repeated pattern of parts and can be found everywhere in nature. Think of the wings of a butterfly, the seeds and leaves in a sunflower or the pattern of a snowflake. Minerals are no exception. In crystals, the shape is determined by the repeated atomic patterns and reflect the 'face' of the crystal. You often see the characteristic symmetry of a crystal with the naked eye, but if the crystal is very small, then a magnifier or magnifying glass is a convenient instrument. Recognizing symmetrical patterns in crystals seems complicated, but experience helps. The more crystals you have seen, the better you can recognize the symmetry. However, sometimes the crystal is far from perfectly shaped and difficult to classify even for experts. In science, crystals are therefore defined by atomic arrangement and not by appearance.
The peculiar properties of quartz crystals
 If you squeeze into a quartz crystal it generates a small electrical current. The pressure on the surface of the crystal forces ions in the crystal to change position, disturbing the balance and turning the crystal into a tiny battery. The phenomenon is known as the piezoelectric effect. This also works the other way around, by putting an electric current on a quartz crystal to make it bend. Quartz watches use this phenomenon to accurately display the time.
If you squeeze into a quartz crystal it generates a small electrical current. The pressure on the surface of the crystal forces ions in the crystal to change position, disturbing the balance and turning the crystal into a tiny battery. The phenomenon is known as the piezoelectric effect. This also works the other way around, by putting an electric current on a quartz crystal to make it bend. Quartz watches use this phenomenon to accurately display the time.
Impurities
 During the formation of a crystal, impurities can occur, which means that the "wrong" type of atom is present in a crystal. For example, a perfect diamond would only contain carbon atoms, but it can also contain a few boron atoms. These impurities change the colour of the diamond to light blue. The same goes for a corundum crystal. The type of impurity that is present during crystal growth determines whether we speak of a Ruby or a Sapphire.
During the formation of a crystal, impurities can occur, which means that the "wrong" type of atom is present in a crystal. For example, a perfect diamond would only contain carbon atoms, but it can also contain a few boron atoms. These impurities change the colour of the diamond to light blue. The same goes for a corundum crystal. The type of impurity that is present during crystal growth determines whether we speak of a Ruby or a Sapphire.
The biggest crystals in the world
 The biggest crystals in the world are located 300 meters below the Mexican town of Naica. In the year 2000 a cavity of 10 by 30 meters was found here. The selenite crystals that occur here are huge. The largest crystal is 12 meters long and has a diameter of 4 meters! The temperature is continuously around 58 degrees. Without special equipment a human can't last more then 10 minutes. It has been calculated that the crystals are about 500.000 years old. With special pumps the cavity is kept dry from water for further study.
The biggest crystals in the world are located 300 meters below the Mexican town of Naica. In the year 2000 a cavity of 10 by 30 meters was found here. The selenite crystals that occur here are huge. The largest crystal is 12 meters long and has a diameter of 4 meters! The temperature is continuously around 58 degrees. Without special equipment a human can't last more then 10 minutes. It has been calculated that the crystals are about 500.000 years old. With special pumps the cavity is kept dry from water for further study.
